IFU Prolactin CE (dal lotto 3997) Elisa kit试验原理:
IFU Prolactin CE (dal lotto 3997) Elisa kit是固相夹心法酶联免疫吸附实验(ELISA).已知待测物质浓度的标准品、未知浓度的样品加入微孔酶标板内进行检测。先将待测物质和生物素标记的抗体同时温育。洗涤后,加入亲和素标记过的HRP。再经过温育和洗涤,去除未结合的酶结合物,然后加入底物A、B,和酶结合物同时作用。产生颜色。颜色的深浅和样品中待测物质的浓度呈比例关系。
试剂盒内容及其配制
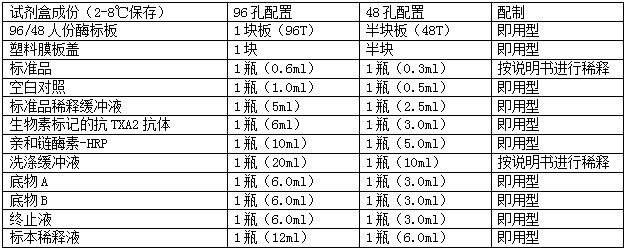
自备材料
1)蒸馏水。
2)加样器:5ul、10ul、50ul、100ul、200ul、500ul、1000ul。
3)振荡器及磁力搅拌器等。
安全性
1)避免直接接触终止液和底物A、B。一旦接触到这些液体,请尽快用水冲洗。
2)实验中不要吃喝、抽烟或使用化妆品。
3)不要用嘴吸取试剂盒里的任何成份。
操作注意事项
1)试剂应按标签说明书储存,使用前恢复到室温。稀稀过后的标准品应丢弃,不可保存。
2)实验中不用的板条应立即放回包装袋中,密封保存,以免变质。
3)不用的其它试剂应包装好或盖好。不同批号的试剂不要混用。保质前使用。
4)使用一次性的吸头以免交叉污染,吸取终止液和底物A、B液时,避免使用带金属部分的加样器。
5)使用干净的塑料容器配置洗涤液。使用前充分混匀试剂盒里的各种成份及样品。
6)洗涤酶标板时应充分拍干,不要将吸水纸直接放入酶标反应孔中吸水。
7)底物A应挥发,避免长时间打开盖子。底物B对光敏感,避免长时间暴露于光下。避免用手接触,有毒。实验完成后应立即读取OD值。
8)加入试剂的顺序应一致,以保证所有反应板孔温育的时间一样。
9)按照说明书中标明的时间、加液的量及顺序进行温育操作。
样品收集、处理及保存方法
1)血清-----操作过程中避免任何细胞刺激。使用不含热原和内毒素的试管。收集血液后,1000×g离心10分钟将血清和红细胞迅速小心地分离。
2)血浆-----EDTA、柠檬酸盐、肝素血浆可用于检测。1000×g离心30分钟去除颗粒。
3)细胞上清液---1000×g离心10分钟去除颗粒和聚合物。
4)组织匀浆-----将组织加入适量生理盐水捣碎。1000×g离心10分钟,取上清液
5)保存------如果样品不立即使用,应将其分成小部分-70
℃保存,避免反复冷冻。尽可能的不要使用溶血或高血脂血。如果血清中大量颗粒,检测前先离心或过滤。不要在37℃或更高的温度加热解冻。应在室温下解冻并确保样品均匀地充分解冻。
试剂的准备
1)标准品:标准品的系列稀释应在实验时准备,不能储存。稀释前将标准品振荡混匀。
2)洗涤缓冲液(50×)的稀释:蒸馏水50倍稀释。
操作步骤
1)使用前,将所有试剂充分混匀。不要使液体产生大量的泡沫,以免加样时加入大量的气泡,产生加样上的误差。
2)根据待测样品数量加上标准品的数量决定所需的板条数。每个标准品和空白孔建议做复孔。每个样品根据自己的数量来定,能使用复孔的尽量做复孔。标本用标本稀释液1:1稀释后加入50ul于反应孔内。
3)加入稀释好后的标准品50ul于反应孔、加入待测样品50ul于反应孔内。立即加入50ul的生物素标记的抗体。盖上膜板,轻轻振荡混匀,37℃温育1小时。
4)甩去孔内液体,每孔加满洗涤液,振荡30秒,甩去洗涤液,用吸水纸拍干。重复此操作3次。如果用洗板机洗涤,洗涤次数增加一次。
5)每孔加入80ul的亲和链酶素-HRP,轻轻振荡混匀,37℃温育30分钟。
6)甩去孔内液体,每孔加满洗涤液,振荡30秒,甩去洗涤液,用吸水纸拍干。重复此操作3次。如果用洗板机洗涤,洗涤次数增加一次。
7)每孔加入底物A、B各50ul,轻轻振荡混匀,37℃温育10分钟。避免光照。
8)取出酶标板,迅速加入50ul终止液,加入终止液后应立即测定结果。
9)在450nm波长处测定各孔的OD值。
局限
6号标准品以上的结果为非线性的,根据此标准曲线无法得到精确的结果。
试剂盒性能
1. 灵敏度:最小的检测浓度小于1号标准品。稀释度的线性。样品线性回归与预期浓度相关系数R值为0.990。
2. 特异性:不与其它细胞因子反应。
3. 重复性:板内、板间变异系数均小于10%。
结果判断与分析
1、仪器值:于波长450nm的酶标仪上读取各孔的OD值
2、以吸光度OD值为纵坐标(Y),相应的待测物质标准品浓度为横坐标(X),做得相应的曲线,样品的待测物质含量可根据其OD值由标准曲线换算出相应的浓度。
酶联生物经过不断的实验优化和改进,积累了大量的经验,拥有专业的酶联研发团队。利用专业的酶联免疫技术自主研发的elisa试剂盒,能对血清及其它样本定量检测抗原,定性检测特异性抗体。优质的试剂,先进的仪器和正确的操作是保证ELISA检测结果准确可靠的必要条件。ELISA检测的方便性、稳定性、重复性和可靠性方面都具有很大的优势。
ELISA检测技术服务内容:
1、双抗体夹心法检测抗原 2、间接法检测抗体 3、为客户提供各种ELISA技术进行样本检测。
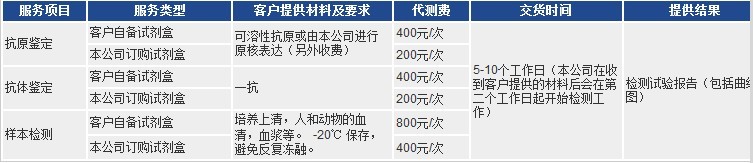
以上代测费,凡购买本公司试剂盒,我们免费代测!
凡购买本公司目录任何一种酶联免疫检测试剂盒,您只需将需要检测的动物(Human, Rat, Mouse, Rabbit, Monkey,
Pig……)种类和检测指标(白介素类、激素类)及标本数量(48T/96T)通知公司业务员即可。在接到客户标本当日起,现货产品一周内将检测报告交到客户手中!
欢迎各科研单位在各种项目上与我们公司开展不同层次的密切合作,以双赢求发展,共同进步,为中国检测事业的发展积累经验。
二、样本要求
在收集标本前都必须有一个完整的计划,必须清楚要检测的成份是否足够稳定。我们提倡新鲜标本尽早检测,对收集后当天就进行检测的标本,及时储存在4℃备用,如有特殊原因需要周期收集标本,请造模取材后,将标本及时分装后放在-20℃或-70℃条件下保存。因冰室与室温存在一定温差,蛋白极易降解,直接影响实验质量,所以避免反复冻融。代测放免标本的客户取材前须向我司销售人员索要说明书,具体操作注意事项请与我司技术人员沟通。
液体类标本:标本必须为液体,不含沉淀。包括血清、血浆、尿液、胸腹水、脑脊液、细胞培养上清、组织匀浆等。
血清:室温血液自然凝固10-20分钟后,离心20分钟左右(2000-3000转/分)。收集上清。如有沉淀形成,应再次离心。
血浆:应根据试剂盒的要求选择EDTA、柠檬酸钠或肝素作为抗凝剂,加入10%(v/v)抗凝剂(0.1M柠檬酸钠或1%heparin
或2.0%EDTA.Na2)混合10-20分钟后,离心20分钟左右(2000-3000转/分)。仔细收集上清。如有沉淀形成,应再次离心。
尿液、胸腹水、脑脊液:用无菌管收集。离心20分钟左右(2000-3000转/分)。仔细收集上清。如有沉淀形成,应再次离心。
细胞培养上清:检测分泌性的成份时,用无菌管收集。离心20分钟左右(2000-3000转/分)。仔细收集上清。检测细胞内的成份时,用PBS(PH7.0-7.4)稀释细胞悬液,细胞浓度达到100万/ml左右。通过反复冻融,以使细胞破坏并放出细胞内成份。离心20分钟左右(2000-3000转/分)。仔细收集上清。保存过程中如有沉淀形成,应再次离心。
组织标本:切割标本后,称取重量。加入一定量的PBS,缓冲液中可加入1μg/L蛋白酶抑制剂或50U/ml的Aprotinin(抑肽酶)。用手工或匀浆器将标本匀浆充分。离心20分钟左右(2000-3000转/分)。仔细收集上清置于-20度或-70度保存,如有必要,可以将样品浓缩干燥。分装后一份待检测,其余冷冻备用。
三、寄标本时需注明以下情况:
1、标本编号;2、所测项目;3、是否做复孔;3、联系方式;4、实验后标本是否寄回。
客户须知:
客户应对所提供的材料及信息负责,如因客户提供的材料及信息不准确而引起的实验延误或经济损失由客户承担。
Q:1.
how to collect samples and preparation of ELISA?
Performed by ELISA test is generally common clinical samples including blood (finger
blood, blood), urine, feces, cerebrospinal fluid, pleural effusion, prostatic fluid,
semen, vaginal secretions, which
Some time of sample collection, preservation methods and has certain requirements.
Collection (a) clinical specimens
A, blood samples:Some physiological factors, such as smoking, eating, exercise, mood
swings, pregnancy, postural changes in blood can affect certain ingredients, even some
of diurnal variation. Therefore, blood samples
Acquisition should avoid interference physiological factors, consistent with appropriate
conditions, such as can not be avoided, should indicate the factors on the specimen.
1. Peripheral:Usually select the inside of blood left ring finger, the portion should be
no frostbite, inflammation, edema, damage. If the site does not meet the requirements to
other parts of the fingers instead. For burn patients, optional leather
Intact skin at the blood. As part of routine blood tests (eg, white blood cell count,
sort, etc.) affected by physiological factors fluctuation is too large, when compared to
the conditional should be consistent. It relates to the body, blood clotting function
Can test items (such as platelet count, bleeding time or clotting time) testing, we must
pay attention to understand whether the patient used anticoagulant, procoagulant drugs
in order to reduce or avoid interfering factors
influences.
2. Blood:In addition to involving a variety of projects such as hemostasis and
thrombosis detector requires the use of anticoagulated blood plasma, the current
analysis to detect the vast majority of projects can be directly detected using blood
serum. In the serum test items
, Some (such as blood sugar, blood fat) diet and circadian factors influenced, fasting
blood samples were generally appropriate; some decay rapidly in the blood (serum enzyme
activity assay such as ACP activity, etc.),
0 ~ 4 ℃ storage is not an activity decreased, the detection of these projects must be
timely and fast; some (such as creatine kinase) influenced by exercise and other
factors. Avoid hemolysis occurs when blood is also important
And, more particularly potassium, LDH and other measurement.
B, urine samples:With the same blood samples, urine samples affect diet, exercise,
medication and other factors that are also large, especially on the diet, so the morning
urine generally superior to random urine. Means getting up early morning urine
After the first urine specimens, representing concentrated and acidified visible
components (such as blood cells, epithelial cells, tubular) easy to observe the relative
concentration. Random urine that is a random urine specimens convenient, but by diet,
Sports, and even more the influence of drugs, prone to false positive and false negative
results, such as diet proteinuria, glucosuria diet, vitamin C interference occult blood
results and the like. Postprandial urine (patient 2 hours after lunch, collected
Human Urine) suitable for urine, urine protein and urobilinogen check urine samples at
this time to increase the sensitivity of the test, the detection of minor lesions. 12
hours in urine cell count is Addis count (last night 8:00
After emptying the bladder to all specimens of urine 8 o'clock the next morning),
because a long time, easy to breed bacteria shall be added preservative formaldehyde.
24-hour urine (the first day of the morning after emptying the bladder specimens from
8:00 to 8:00 the next morning
All urine) quantification of chemical substances, including proteins, sugars, urinary
17-one, 17-hydroxy steroids, catecholamines, Ca2 +, etc., to detect different
substances, choose a different preservative preservative. clean
Urine used for urine bacterial culture requires sterile specimens were taken after
washing the vulva. Urine specimens should be enough to collect all, at least 12 ml,
preferably 50 ml, the timing must collect all the urine of women
Patients should avoid vaginal secretions, blood contamination of urine specimens.
C, stool samples:Stool samples for the detection judgment digestive diseases has
important reference value. Collection requirements with a clean bamboo select faecal
mucus, pus and blood components and other abnormality, no abnormal appearance
Droppings shall be drawn from multiple surface and deep manure end. Get parasitemia and
for egg counts should be collected 24 hours feces. Dysentery amoeba trophozoites check
should immediately check in after a bowel movement, and from there sepsis
Softer at the drawn, insulation inspection. Charles S. japonicum eggs should take mucus,
pus and blood portion 30g stool specimens from at least miracidia hatching, and to be
treated as soon as possible. Check pinworm eggs must use transparent film swab
Night before 12:00 or early in the morning from defecation wrinkled folds around the
anus and immediately swabbing at microscopic examination. Occult blood test (chemistry),
fasting before the test on the 3rd of meat and foods containing animal blood and ban
clothing iron, vitamin C and so on.
Should be checked in all 1 hour stool specimen collection is completed, in order to
prevent damage to physical components of digestive enzymes and pH by. For clinical
samples above the detection indicators.
D, CSF samples:CSF samples collected immediately after submission, place too long will
affect the test results: such as cell degeneration, destruction, leading to counting and
classification are not allowed; some chemicals such as glucose content will decompose
Save
Less; bacteria occur autolysis affect bacteria detection rate. Cerebrospinal fluid
extracted three general dispensing a sterile tube, the first tube for bacterial culture,
a second tube for chemical analysis and immunological tests, the third tube for general
Characters and microscopic examination, three of the order should be reversed. Specimen
collection is difficult because all inspection and testing process should pay attention
to safety.
E, ascites and pleural effusion samples:CSF samples with the same attention to safety
after the specimen collection, and timely submission. Generally separated into three
tubes, one for routine cytology, a biochemical examination, a bacterial culture, in
order
CSF same is appropriate.
F, prostatic fluid sample:Prostatic fluid specimen after prostate massage by the
acquisition, directly drop when less liquid on a glass slide and timely submission shall
be taken to prevent sample evaporation to dryness, the amount collected for a long time
in a clean, dry test tube. If massage
No prostatic fluid, urine sediment can be checked after the massage.
G, semen samples:Abstinence before semen collection should be 3 to 7 days, drain the
urine after masturbation or other available methods of semen directly into clean
containers, insulation and timely submission. Due to changes in sperm production during
the day and
Large, generally should be checked 2 to 3 times (each time interval of 1 to 2 weeks) in
order to make a diagnosis.
H, samples of vaginal secretions:Vaginal samples were collected 24 hours before
intercourse should be prohibited, bath, vaginal examination, vaginal lavage and local on
the drug, etc., drawing instruments used need to be cleaned. Usually with brine-soaked
cotton swab from the vagina deep
Or rear vaginal fornix, cervical canal mouth drawn, etc., made after saline smear
vaginal secretion samples observation, women with menstrual vaginal secretions were not
checking.
2, do before each sample by ELISA experiment how to prepare?
Before collecting the sample must have a comprehensive plan must clearly be detected
component is stable enough. To be collected on the same day
Sample testing, and timely backup stored at 4 ℃. For the next day re-testing samples
frozen in a timely manner after dispensing -20 ℃ spare, conditional, preferably -70 ℃
cryopreservation standby. Avoid repeated freezing and thawing specimens
.
Liquid samples: including serum, plasma, urine, pleural effusion, cerebrospinal fluid,
cell culture supernatant and the like.
1. serum:
Coagulation at room temperature 10-20 mins, centrifugation 20 minutes or so (2000-3000
rev / min). Carefully collect the supernatant. If precipitation during storage,
Centrifugal again.
2. Plasma:
EDTA should be selected according to the requirements of the specimen, sodium citrate or
heparin as an anticoagulant, mix 10-20 mins, centrifugation 20 minutes or so (2000-3000
rev / min). Carefully collect the supernatant. Save process
If precipitation appeared, Centrifugal again.
3. Urine:
Sterile collection tube. Centrifuged for 20 minutes or so (2000-3000 rev / min).
Carefully collect the supernatant. If precipitation during storage, Centrifugal again.
Pleural and peritoneal effusions, and cerebrospinal fluid Reference to this practice.
4. The cell culture supernatant:
The detection of secretory component with a sterile collection tube. Centrifuged for 20
minutes or so (2000-3000 rev / min). Carefully collect the supernatant.
5. cultured cells
????When the detection of intracellular components, diluted with PBS (PH7.2-7.4) cell
suspension, the cell concentration reached 1 million / ml or so. By repeated freezing
and thawing or tissue protein extraction reagent was added to the cells
Damage and release of intracellular components. Centrifuged for 20 minutes or so
(2000-3000 rev / min). Carefully collect the supernatant. If precipitation during
storage, Centrifugal again.
6. tissues
????After cutting samples, check the weight. Adding a certain amount of PBS, PH7.4.
Rapidly frozen with liquid nitrogen. After thawing samples remained at 2-8 ℃. Adding a
certain amount of PBS
(PH7.4), or tissue protein extraction reagent, or by hand homogenizer homogenized
sample. Centrifuged for 20 minutes or so (2000-3000 rev / min). Carefully collect the
supernatant. A new package to be detected, which
I alternate freezing.
Q:Do
I have to run all of my standards and samples in duplicate?
A:Yes, the duplicates are run in order to monitor assay precision and increase
confidence in the assay results obtained.
Q:Do
I have to run all of my samples at one time?
A:No, each kit uses stripwell microplate. This allows the user to analyse different
numbers of samples at different times.
Q:What
types of reproducible results are obtained with the assays?
A:Each kit comes with a manual containing a graph of typical data obtained. Any
variation in operator, pipetting and washing technique, incubation time or temperature,
and kit age can cause variation in result. Each user should obtain their own standard
curve.
Q:Is
it possible to store the reagents other than indicated?
A:Storage of the kit components under conditions other than indicated is not recommended
in order to assure proper performance of the test.
Q:How
should I store my samples?
A:Samples should be stored at -20oC or lower temperature. For long-term storage, it is
recommended to freeze them at -70oC -80oC.
Q:Can
I modify the protocol?
A:BG ELISA kits have been optimized to provide the best possible results. Modifying the
format or protocol may give inaccurate and wrong results.
Q:Can
I use a sample type that is not recommended in the kit insert?
A:The kit has been validated for the sample types listed in the kit insert. Sample types
other than those validated have not been tested. Contact Technical Service for further
information.
Q:My
samples generated values that were outside the dynamic range of the assay. Can I use
these values?
A:It is recommended that only sample values that fall within the range of the standard
curve be used. Values outside the range of the standard curve are generally non-linear,
which can lead to incorrectly extrapolated values. Samples that generate values higher
than the highest standard should be (further) diluted and the assay repeated. If samples
fall below the range of the assay, the sample is considered to be non-detectable.
Q:Do
I have to run a Blank or Zero Standards every time?
A:Yes, these are required for the calculations, and reflect any subtle but significant
performance changes from day to day and assay to assay. They are also extremely helpful
when troubleshooting the source of a particular assay problem.
Q:Can
I alter the volume of sample I use in the assay?
A:It is not recommended that you alter the volumes since all BG kits are designed for
optimal performance at the given volumes
Q:Can
components from different kits be used?
A:Each kit contains components which have specific lot numbers to ensure that all of the
components are performing optimally alone, as well as with all of the other components
in the kit. QC testing is performed on these specific lots. It is never recommended to
use your own components or components from other kits or vendors.
Q:My
standard curve looked fine, but I didn’t get a signal in my sample when I expected
to, why?
A:The sample may not contain the analyte. A matrix effect may be masking the detection.
Ensure that the recommended dilution was followed as stated in the kit insert. If
dilution was recommended, check to be sure that the dilution was performed properly.
Over-dilution may cause the sample to fall below the range of the standard curve.
Q:How
do you recommend I wash my plate?
A:If you are using an automated plate washer we recommend that the calibration be
checked on a regular basis, and that the system is flushed with the Plate Washing Buffer
prior to washing. The same is true for a manual washer. A repeater or a wash bottle can
also be used. The user should be careful to ensure that all of the contents are
aspirated and the plate tapped dry on lint-free paper.
Q:Do
I need to use a plate shaker?
A:Reliable results can be obtained without a plate shaker, but the O.D.'s will generally
be lower than those obtained using a plate shaker.
Q:Why
do I have to use wavelength correction between 450-570nm?
A:For the ELISA assay, reading at dual wavelengths is done to correct for the optical
density contributed by the plastic well, the lamp and optical fluctuations.
Q:If
I extract my sample, do I still need to follow the recommended dilutions given in
the kit insert?
A:The amount of sample dilution needed after an extraction procedure will be affected by
the effects of purification and concentration in the protocol used. The amount of
dilution or concentration will have to be determined by the end-user.
Q:What
is the expected concentration of analyte that I should expect to find?
A:The amount of a given analyte may vary not only from species-to-species, but also
between tissue and cellular sources. The best source of this information is the current
literature that is easily accessed through the Internet at multiple scientific
databases.
Q:My
optical densities were a little higher (or lower) than those in the manual that came
with my kit. Why?
A:The optical density is affected by a number of physical conditions such as time and
temperature. We suggest that you shorten or lengthen the final incubation with substrate
solution to compensate.
Q:What
are the reasons for High Background?
A:1) Improper Washing: Check volume of washing buffer reservoir and make sure all
recommended washing steps are performed.
2) Contaminated Substrate: Make sure there is no contamination of the substrate with
metal ions or oxidizing reagents, before use. Keep the extra substrate solution
separately during the ELISA substrate development time.
3) Substrate exposed to light: Exposure to light may result in a blue color of the
substrate. Keep solutions in the dark (vial) until ready to dispense into the plate.
4) Wrong Incubation Times/Temperatures: Generally follow the test protocol regarding
incubation times and temperatures. However, if all wells are intensely and equally
colored with no intensity gradient observed in the standard dilution series, then it may
be necessary to observe the substrate reaction as the color is developing, in order to
stop the reaction sooner.


 酶联官方手机二维码
酶联官方手机二维码










